We would like to hear from you with any feedback about our website or products.
Briefing of EU Food Contact Legislation
As a professional food service supplier, we try our best to understand everything in this vertical domain to help our customers. Today, we would like to share briefing of EU food contact legislation.
Why EU?
The reason is EU has advanced legislation, which follows industrial changes. Besides, because of the complex union relationship, there are slight differences between different countries. We will give a concept about EU legislation, then go through 3 major EU members. Once we understand EU’s legislation, it’s also easier to understand other countries with similar structure, like Australia, China, India, south America (MERCOSUR) and so on.
EU Legislation Architecture
To understand EU food contact legislation, we begins from Regulation (EC) No 1935/2004, it provides us a framework to follow with basic requirement but no specific limitation or numbers. Under 1935/2004/EC, there are directives those provides testing methods, conditions and criteria. For food contact field in TRENDWARE Products, normally we focus on Commission Regulation (EU) No 10/2011 (plastic) and Council Directive 84/500/EEC (ceramic), also Commission Directive 2004/14/EC (regenerated cellulose file).
Besides, there are guidelines and recommendations which provide policy statements, research documents, but they are not legally binding. Also, if you want to know the current level of science and technology for the conditions under which food contact materials and substance can meet the requirements for Regulation No (EC) 1935/2004. You can refer to this Eupropean Commission page for more.
Food Contact Coverage
1935/2004/EC article 1 mentioned:
This Regulation shall apply to materials and articles, including active and intelligent food contact materials and articles, (hereinafter referred to as materials and articles) which in their finished state:
- are intended to be brought into contact with food; or
- are already in contact with food and were intended for that purpose; or
- can reasonably be expected to be brought into contact with food or to transfer their constituents to food under normal or foreseeable conditions of use.
That is to say, while preparing your product for test, you should consider all material will contact to all possible condition; otherwise, you might fail. For example, if you have a cutting board with padding other than the surface, the padding should be tested since it’s possible to contact with food.
Food Contact Testings
According to 1935/2004/EC article 3, all food contact materials require:
- Must not endanger human health
- Must not bring about an unacceptable change in the composition of the food
- Must not bring about a deterioration in the organoleptic (smell and taste) characteristics hereof
These requirements map to these tests:
- Sensorial testing
- Overall migration, maximum permitted about of non-volatile substances released from a material or article into food simulants
- Specific migration, maximum permitted amount of a given substance released from a material or article into food or food simulants
- NIAS (Non-intentionally added substance)
For the first 3 tests, you can request test conditions which fit to your product user scenario, with given food simulant, temperature and time, then get a pass or fail result.
But NIAS is different, it means an unexpected result during your manufacture process. As it’s not expected, the way to test NIAS seems ambiguous. NIAS should be assessed by the manufacturer in accordance with internationally recognised scientific principles on risk assessment. They will go through the raw material and process to know if there is any risk in your final product state.
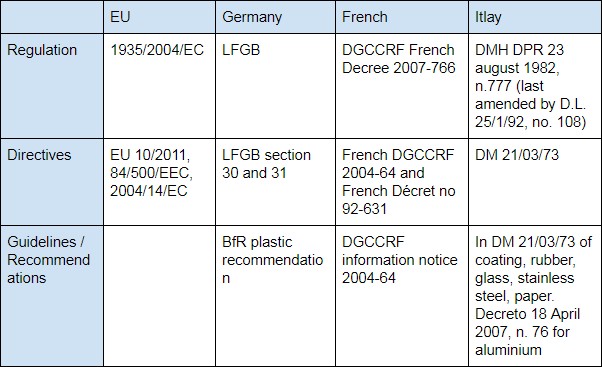
Domestic Legislation
As described above, 1935/2004/EC is an EU legislation as a framework for countries to follow. In Germany, French and Italy, the 3 major EU members, besides 1935/2004/EC, import food contact products also need follow legislation of:
- Germany: LFGB (Lebensmittel-, Bedarfsgegenstände- und Futtermittelgesetzbuch)
- French DGCCRF (the French General Directorate for Competition Policy, Consumer Affairs and Fraud Control,)
- Itlay: Italian Decree of the Ministry of Health (DM / DMH, No. 283 of 30/04/1962 & D.M.21/03/73)
- United Kingdom: UK SI 898: 2005 before Brexit.
RASFF Portal
We tell a lot about EU legislation, and importers should be responsible for their products. However, goods ship to EU only take random check in customs. Once not qualified, EU can ask you to do actions, like recall from consumers or even withdrawal from the market. IMHO, DO NOT take the risk. See cases on RASFF Portal, it also lets you know how EU react to that.
In Product form, select “food contact materials” in Category, then click “Get results”, you can see these cases.
Thanks to TÜV Rheinland Taiwan’s seminar for this post. If you have any question, please feel free to contact us.

 繁體中文
繁體中文  Español
Español  Русский
Русский 
